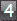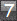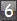In group 1 there was 1 oligodendroglioma (0.8%): in group 2 there
was 1 ependymoma and 1 astrocytoma (1.7%): in group 3, 1 astrocytoma
(0.8%), and in group 4, 1 oligodendroglioma (0.8%). There are two facts
which require consideration. The first is that the studies were
performed by a manufacturer of aspartame. The second is what is meant
by an atypical astrocytoma observed in a control animal, yielding an
incidence of brain tumor comparable to the experimental groups. Also,
it should be noted that there were 2 brain tumors in group 2, doubling
the incidence in comparison with the other groups. This experiment
should have raised appropriate concern leading to further study.
Discussion
Presentation of this epidemiologic hypothesis is predicated upon (a) the
animal data, and (b) the progressive increase in incidence of several
types of primary brain cancer within one or two years after the
marketing of aspartame in both "dry" and "wet" products - namely, 1982
data on primary brain lymphoma, and 1984-1987 data on glioblastoma and
astrocytoma. In this regard, Altschule (14) aptly noted that a good
hypothesis must bring together scattered data or explain a previously
unexplained phenomenon.
These phenomena have not been convincingly explained on the basis
of improved diagnostic methods or other factors. The latter include
decreased immunocompetence associated with HIV infection, other viral
disorders, overall trends in non-Hodgkin’s lymphoma, occupational
exposure (15), or specific chemical exposures (2,4,16). For example, a
25-year registry of patients with non-Hodgkin’s lymphoma, sarcoma,
leukemia and aplastic anemia ascribed to occupational or home exposure
to pentachlorophenol and its dioxin-dibenzufuran contaminants (17)
failed to suggest any increase of central nervous system malignancies.
Similarly, Krinke et al (18) could find no statistically significant
evidence for an increased incidence of brain tumors in rats exposed to
14 different types of long-term or life-span studies. They were
conducted to determine the carcinogenicity of many drugs, agrochemicals,
dyestuffs, plastics, and other industrial chemicals. These
investigators also confirmed that neurological tumors occurred
spontaneously in only 11 among 8960n aging Sprague-Dawley rats.
A recent review of the value of chemical carcinogenicity studies on
laboratory animals by Huff, Haseman and Ral (19) is germane. These
authorities underscored the value of laboratory rodents as the key
surrogates for presently identifying carcinogenic chemicals in humans.
They emphasized the prudent public health responsibility of regarding
evidence of experimental carcinogenicity as a plausible basis for
inferring a carcinogenic risk in man when adequate human data are not
available. In the absence of sufficient and reliable human data, they
also anticipated social and political debate when chemicals with
economic importance are shown to induce experimental cancer.
Brain Cancer in Females
The apparent increase of these tumors in women is relevant. It has been
generally acknowledged that malignant brain tumors in adults occur more
often among men (5,15,16). Older male rates also develop more
spontaneous brain tumors than females (18).
The increase of fatal brain cancer among women is illustrated by
the following death rates per 100,000 populations among females of all
ages (Silverberg, E., Department of Epidemiology & Statistics, American
Cancer Society, Personal Communication, March 20, 1990): 1979--3.4;
1980--3.5; 1981--3.5; 1982--3.6; 1983--3.6; 1984--3.8; 1985--3.8;
1986--3.8; 1987--4.0.
These increases were more striking among white than non-white
women. Socioeconomic and cultural factors pertaining to the consumption
of "diet" drinks during the early 1980s might partly explain these
discrepancies. For example, the increased incidence of gliomas in Jews
could also be correlated with increasing socioeconomic class in a Los
Angeles County study (20). The threefold higher incidence of severe
reaction to aspartame products in females compared with males (7,8) is
also germane.
Admittedly, the writer’s personal data base concerning this problem
remains limited. For example, the 49-year-old wife of a physician
developed fatal cerebellar glioblastoma. As a weight-conscious actress
and television personality, she had consumed considerable amounts of
aspartame products. She had enjoyed good health previously and did not
smoke.
Anecdotal information indicates the potential for bone marrow
stimulation by aspartame products. Case examples include eosinophilia
in an adult female, and three leukemoid episodes in a girl diagnosed as
histiocytic leukemia after repeated aspartame rechallenge (21).
There have been other related observation. During 1987, Roelvink
et al (22) reported four patients in whom a primary malignant brain or
spinal tumor first became manifest during pregnancy. Their interest was
prompted by encountering them within a relatively short time period.
They were unable to attribute such neoplasms, however, to hormonal
influences of gestation.
Pathogenic Insights
Caldecott (23), a member of the Atomic Energy Commission, warned that by
far the most mutagenic agents known to man are chemical, not radiation.
He suggested that food additives may pose a greater danger than present
levels of fallout. Confirmation of these epidemiologic relationships by
others might suggest new approaches to the etiology and pathogenesis of
primary brain tumors.
Aspartame and its components or its metabolites might activate one
or more oncogenes that potentiate or initiate cell mitosis (2), either
by direct or indirect effects -- for example, tissue glucopenia, or the
influence of uncommon amino acid dextroisomers. These oncogenes include
c-cis, c-er B, N-ras, c-myc, the epidermal growth factor-receptor (EGF-
R) gene (24), and the trk proto-oncogeny receptor for nerve growth
factor (25).
Another mechanism might be the substitution of no-calorie or low-
calorie products for conventional foods and beverages, whether as meals
or snacks. This can have serious sequelae in the brain, a point
emphasized in prior publications concerning the pathogenesis of multiple
sclerosis (26), narcolepsy (27), seizures (27), and migraine (28).
Under normal circumstances, the brain is almost totally dependent upon
glucose for optimum function.
The initial rise in incidence of primary brain lymphoma in 1982,
when the consumption of aspartame was much less than after its "wet" use
approval during 1983, is of interest. It might be explained by the
influence of a less intense biophysiologic, immunologic, viral or
toxicologic stimulus than for the more common types of brain tumor. It
is noteworthy that the central nervous system (CNS) generally lacks a
lymphatic circulation and endogenous accumulations of lymphoid tissue.
Hochberg and Miller (5) suggested that an unknown second event in the
local site or sites than transforms a clone of the inflammatory cell
population into neoplastic cells. Another scenarious might be the
migration of activated "homing" cells or molecules from B lymphocytes
elsewhere that carry a CNS-specific binding marker.
An unchecked hyperinsulinized state also appears to be operative in
the pathogenesis of other tumors. Prostatic hyperplasia and neoplasia
(29) provide examples. Phenylalanine and aspartic acid, the amino acid
components of aspartame, are known to stimulate insulin release (30-32).
Recommendations
The relationship between aspartame consumption and the development of
primary brain cancers in humans requires careful analysis by corporate-
neutral investigators. In the event that such a correlation is shown
and brain cancer incidence rates continue to rise, the FDA should
declare aspartame products and "imminent public health hazard".
Other findings would have related significance. They include:
(a) A disproportionate increase in incidence of glioblastoma,
astrocytoma and primary brain lymphoma among young women who, generally
speaking, are known to be consuming considerable amounts of aspartame.
Brain tumors heretofore have occurred predominantly in middle-aged men
(20).
(b) An increase in the incidence rates of gliomas among children whose
mothers consumed aspartame during pregnancy. Phenylalanine concentrates
at least fourfold on the fetal side of the placenta (7).
Many reasons already exist for such a declaration by the FDA. This
agency has received an unprecedented number of volunteered complaints
from at least 5,000 consumers concerning severe reactions attributed to
aspartame products - including 250 cases of convulsions. The author’s
registry of 630 aspartame reactors contains more than 100 individuals
with grand mal and other seizures. The list of other central nervous
system reactions to aspartame products is long (7,8), again indicating
that serious brain dysfunction can be induced or aggravated by these
products. In this context, I have urged that a formal diagnosis of
multiple sclerosis be delayed in persons consuming aspartame products
pending their observation for one or several months after abstinence.
References
1. National Cancer Institutes. Cancer Statistics Review 1973-87.
Bethesda, NIH Publication No. 89-2789.
2. Black, P. McL: Brain tumors, New Engl. J. Med 1991: 324:1471-1476.
3. Hochberg F. Toniolo P, Cole P: Nonoccupational risk indicators of
glioblasoma in adults, J Neuro-Oncol 1990; 8:55-60.
4. Eby NL, Grufferman S. Flannelly CM. et al: Increasing incidence of
primary brain lymphoma in the US. Cancer 1988; 62:22461-22465.
5. Hochberg FH, Miller DC: Primary central nervous system lymphoma. J
Neurogurg 1988: 68:835-853.
6. Hardwidge C, Diengdoh JV, Husfaud D. Nash JRG: Review: Primary
cerebral lymphoma - a clinico-pathological study. Clin Neuropath 1990;
9:217-223.
7. Roberts HJ. Aspartame (NutraSweet): Is It Safe? Philadelphia The
Charles Press, 1989.
8. Roberts HJ. Reactions attributed to aspartame-containing products:
551 cases. J Appl Nutr 1988: 59:85-94.
9. Congressional Recond-Senate. Saccharin Study and Labeling Act
Amendments of 1985. May 7, 1985, pp.S5489-5516.
10. Congressional Record-Senate. Aspartame Safety Act of 1985. August
1, 1985, pp.S10820-10847.
11. U.S. Government Printing Office. Aspartame; Availability of Board
of Inquiry Decision. Fed Reg 1980; 45:69558.
12. Community Nutrition Institute, et al. v. Dr. Mark Novitch, Acting
Commissioner, Food and Drug Administration, United States Court of
Appeals for the District of Columbia Circuit, No. 84-1153 and No. 84-
5253 (D.C. Civil Action No. 83-03846, decided September 24, 1985).
13. Ishu H.: Incidence of brain tumors in rats fed aspartame. Toxicol
Letters 1981: 7:433-437.
14. Altschule MD: Hypothesis in medicine. Med Sci 1966; 17:94
15. Salcman M: The morbidity and mortality of brain tumors, Neurol Clin
1985; 3:229-257.
16. Cole GC, Wilkins PR, West RR: An epidemiological survey of primary
tumors of the brain and spinal cord in South East Wales. Br J Neurogurg
1989; 3:487-493.
17. Roberts HJ: Pentachlorophenol-associated aplastic anemia, red cell
aplasis, leukemia and other blood disorders. J Florida M A 1990; 77:86-
90.
18. Krinke G. Naylor DC. Schnid S. Frohlich E. Schnider K: The
incidence of naturally-occurring primary brain tumors in the laboratory
rat. J Comp Path 1985; 95:175-192.
19. Huff J. Haseman J. Rall D: Scientific concepts, value, and
significance of chemical carcinogenesis studies. Ann Rev Pharmacol
Toxicol 1991; 31:621-652.
20. Preston-Martin S: Descriptie epidemiology of primary tumors of the
brain, crainial nerves and cranial meninges in Los Angeles County.
Neuropidem 1989; 8:283-295.
21. Roberts HJ: Aspartame, tryptophan, and other amino acids as
potential hazardous experiments. South M J 1990; 83:1110-1111.
22. Roelvink NCA. Kamphort W, van Alphen, HAM. Rao BR: Pregnancy-related
primary brain and spinal tumors. Arch Neurol 1987: 44:209-215.
23. Caldecott R: Cited by Sc Newsletter 1961: November 18.
24. Hoi Sang U. Kelley PY, Hatton JE, Shew JY: Proto-oncogene
abnormalities and their relationship to tumorigenicity in some human
gliblastomas. J Neurosurg 1989: 71:83-90.
25. Kaplan DR, et al: The trk proto-onogeny product: A signal
transducing receptor for nerve growth factor. Science 1991:252-554-558.
26. Roberts HJ: An inquiry into the pathogenesis, rational treatment
and prevention of multiple sclerosis, with emphasis upon the combined
role of diabetogenic hyper-insulinism and recurrent edema. J Am Geriat
Soc 1964; 12:926-976.
27. Roberts HJ: The syndrome of narcolepsy and diabetognenic
("function") hyperinsulinism, with special reference to obesity,
diabetes, idiopathic edema, cerebral dysrhythmias and multiple sclerosis
(200 patients), J Am Geriat Soc 1964; 12:926-976.
28. Roberts HJ: Migraine and related vascular headaches due to
diabetogenic hyperinsulinism: Observations of pathogenesis and rational
treatment in 421 patients. Headache 1967; 7:41-62.
29. Roberts HJ: Pathogenesis of prostatic hyperlasia and neoplasia.
Geriat 1967; 22:85-92.
30. Floyd JC Jr., Fajans S. Conn JW, Knopf RF, and Rull J: Stimulation
of insulin secretion by amino acids. J Clin Invest 45:1487-1502. |
|